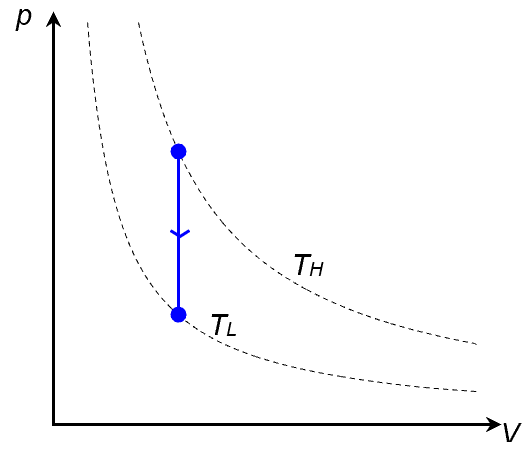
Isochoric literally means constant volume. On the P-V diagram, an isochoric cooling is represented by a downward vertical line: vertical because volume is constant, downward because it must move from a higher isotherm to a lower isotherm.
Since cooling implies a decrease in temperature, . And since the volume does not change,
.
Applying the first law of thermodynamics,
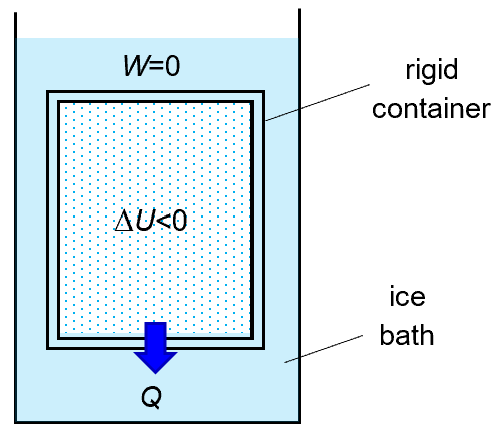
it is also clear that Q is negative, i.e. heat is lost to the surrounding during an isochoric cooling.
A simple practical example of an isochoric cooling is to immerse a canister (with rigid walls) of gas in an ice bath.
–