Decay Constant
The very fact that radioactive decay is spontaneous and random means that the activity A of a radioactive sample must be proportional to the number of undecayed nuclei N in the sample. This is the basis for the formula
The constant of proportionality λ is called the decay constant. It is often defined as “the probability per unit time that a nucleus decays”. When λ has a small numerical value (this depends on the chosen time unit), it is indeed approximately equal to the probability of decay during the chosen time unit. However, when λ has a large numerical value, the number does not have much meaning. For example, a decay constant of 0.01 day-1 does imply that the probability that a nucleus decays in one day is (approximately) 0.01. Note that 0.01 day-1 can also be expressed as 3.65 yr-1. But 3.65 does not have any physical meaning.
Example
The alpha decay of radon-222 into polonium-218 has a decay constant of . Calculate
a) the probability that a radon-222 nucleus decay in 1 min.
b) the activity of a sample containing 2 billion undecayed radon-222 nuclei.
Solution
a)
The probability of that a nucleus decays in 1 minute is .
b)
–
Exponential Decay
If a radioactive sample contains N0 number of undecayed nuclei at , then number of undecayed nuclei N at time t is given by
This is called an exponential decay. The larger the decay constant λ, the faster the sample is depleted.
How does this exponential relationship come about? Well, we start from the fact that activity is proportional to the number of undecayed nuclei.
Since the activity is also equal to the rate at which the number of undecayed nuclei decreases,
Integrating both sides,
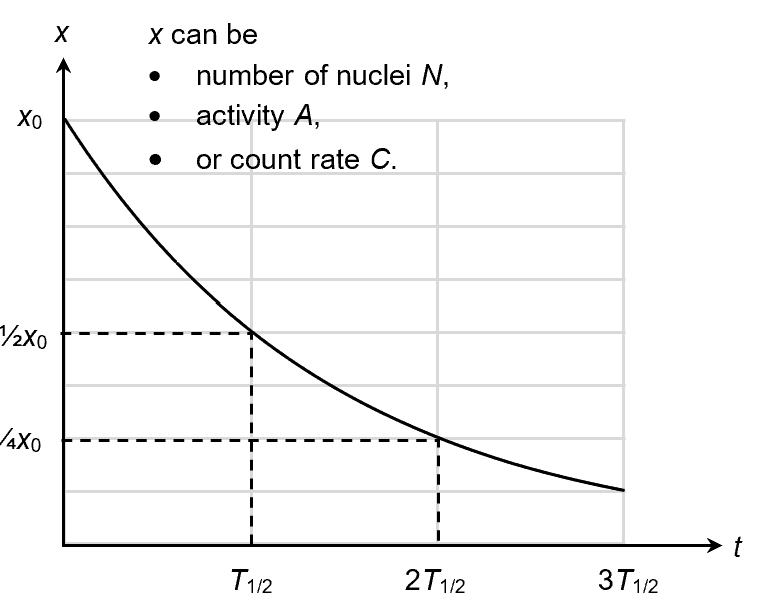
N is not the only thing that decays exponentially. Since the activity A is proportional to the number of undecayed nuclei ( and
), A should also decrease exponentially over time.
In practice, the activity is measured using a Geiger-Muller (GM) counter. Since the count rate C is proportional to the activity, C should also decrease exponentially with time.
Besides the decay constant, the half-life, T1/2, is the other parameter that is used to characterize the rate of decay of a nuclide. It is the (average) time taken for half of the nuclei in the population to decay. You can also think of it as the (average) time taken for a sample’s activity to be halved. The relationship between decay constant and half-life can be derived as below.
For example, Rn-222, which has a decay constant of , has a half-life of
.
The cute thing about an exponential decay is that the quantity falls by the same percentage given the same amount of time. So it does not matter when we start counting. The number of undecayed nuclei N in a sample of Rn-222 number is halved after every 3.8 days.
Before we leave this section, let’s remind ourselves that the exponential formulae are accurate only if N is large. Radioactive decay is at its core a random event. In practice, there will always be random fluctuations in the measured count rate that result in deviations from the theoretical values.
–