Intensity and frequency of light as a wave

According to the wave model of light, the difference between a bright and dim light is in the amplitude of the wave.

And the difference between light of different color is in the wavelength (or frequency).
–
The Wave model’s predictions

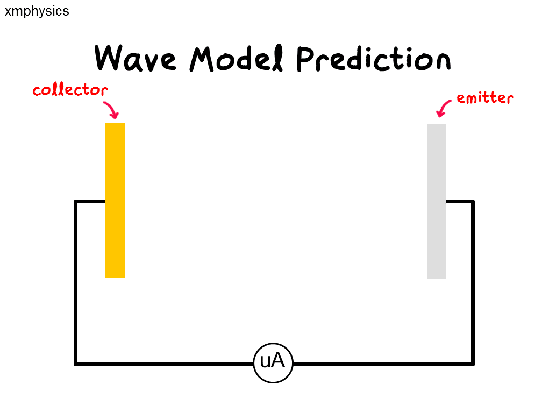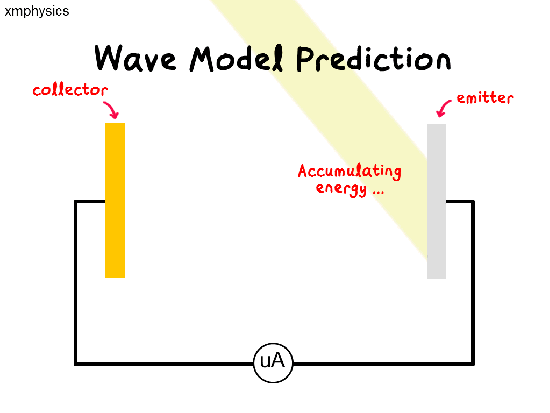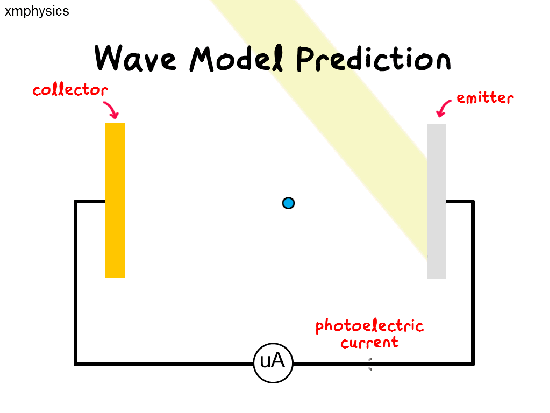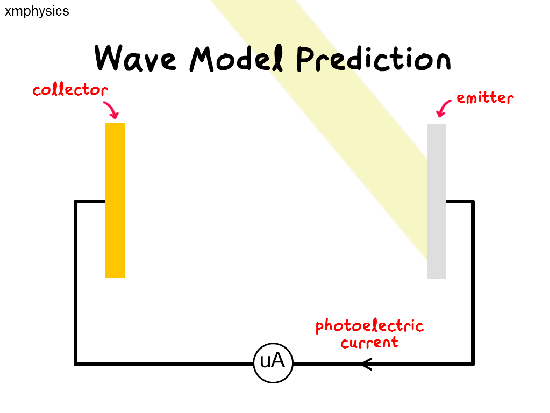
If light is a wave, a beam of light illuminating the metal surface would be delivering energy to trillions of electrons simultaneously at the same time. The electrons would have to share and accumulate the energy over time, before they have enough energy to be liberated from the metal. So there should be a time lag between illumination and emission.
Remember that a wave’s power is proportional to the amplitude-square of the wave. So whatever the frequency of light, we should be able to increase the rate of supply of energy to the electrons by increasing the intensity of the light.
So regardless of the frequency of light used, as we increase the intensity of light, we expect (1) more photoelectrons to be emitted per unit time (higher rate of emission), (2) more energetic photoelectrons (higher KEmax) and (3) shorter time lag between illumination and emission.
–
Actual experimental observations
Experimental results however produce 3 contradictions.
(1) Increasing the intensity of light increases the saturation current but not the stopping potential. The stopping potential increases only when higher frequency light is used. (The wave model predicts that KEmax should increase with intensity of light).
(2) If the frequency of light used is below a certain threshold frequency, the photoelectric current will still remain at zero regardless of the intensity of the light. (The wave model predicts that photoelectric effect should occur as long as the intensity is high enough, regardless of frequency)
(3) The microammeter registers a (small) current immediately after the light is switched on, even when very low intensity light is used. (The wave model predicts that there should be noticeable time at low intensity when the rate of supply of energy is low)
–
Three observations that show light is not a wave
In summary, the following are the three experimental observations that cast doubt on the wave model of light. Eventually, these three observations will provide evidence for the particulate nature of light.
- KEmax is dependent on the frequency, but indepenent of intensity of light.
- Photoelectric effect does not occur below the threshold frequency, regardless of intensity.
- There is zero time lag even at low intensity.